Root nodule symbiosis
Some plants like legumes can form specialized organs called root nodules, allowing them to symbiotically associate with nitrogen-fixing rhizobia. Through this root nodule symbiosis (RNS), plants receive nitrogen nutrients from rhizobia while providing them with photosynthetic products as an energy source. The establishment of this symbiosis involves several key processes, including the infection of roots, the initiation of nodule formation, the development of nodules, and the driving of nitrogen fixation, all of which are genetically controlled by both plants and rhizobia. Since around 2000, many related genes have been identified, leading to a better understanding of the symbiotic mechanisms; however, many aspects remain unresolved, and new questions continue to arise as research progresses.
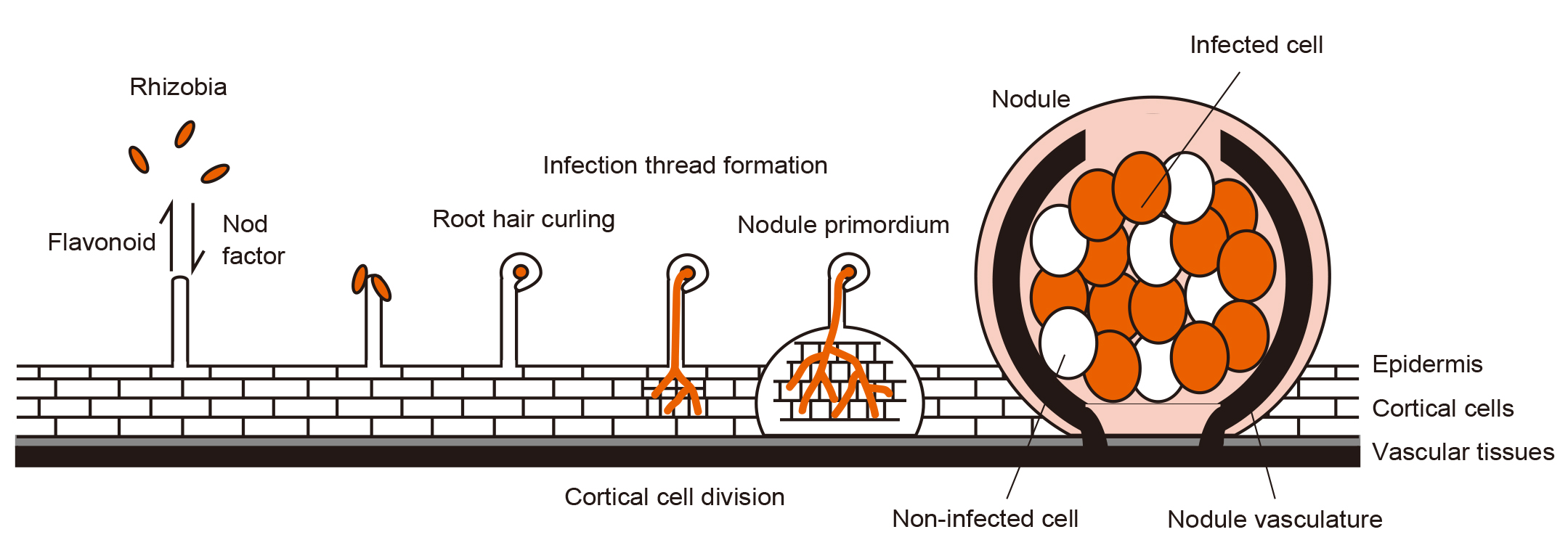
Root nodule symbiosis begins with the exchange of signaling molecules between the plants and rhizobia. Following this, the infection of rhizobia and their invasion into the roots, as well as the nodule organogenesis, proceed in a coordinated manner. The rhizobia within the nodule cells perform nitrogen fixation.
Research Summary
Mechanisms controlling root nodule symbiosis in response to environmental changes
RNS benefits plants by supplying nitrogen nutrients but is sensitive to environmental changes. Our research group focuses on understanding how nitrogen levels control this symbiosis. When sufficient nitrate is present, plants temporarily inhibit RNS, shifting from nodule-derived nitrogen to soil nitrogen. To elucidate the underlying mechanisms, we have been using the model legume plant Lotus japonicus for our studies, identifying several nitrate unresponsive symbiosis (nrsym) mutants that maintain RNS even in high nitrate conditions. Key findings include the role of the NRSYM1 gene, which encodes the LjNLP4 transcription factor that suppresses nodule formation via a systemic signaling pathway involving the CLE-RS2 peptide (Nature Commun 2018). We further identified another NLP transcription factor, LjNLP1, and a nitrate transporter LjNRT2.1; they act in the same genetic pathway with LjNLP4 (Plant Cell 2021, 2022). We are now aiming to identify further components of this regulatory system.
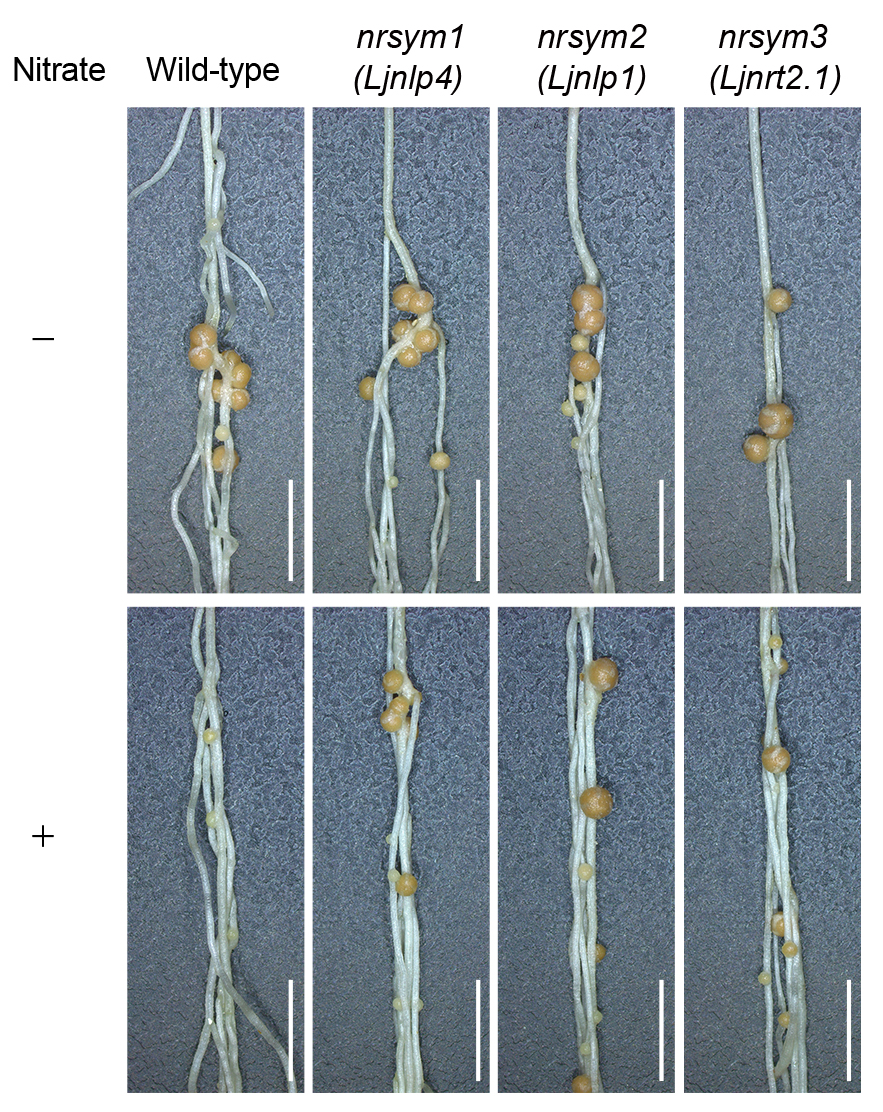
The nrsym mutants of Lotus japonicus form functional nodules even under conditions of high nitrate availability. Scale bars: 5 mm.
Evolutionary basis of root nodule symbiosis
Uncovering the mechanisms underlying the emergence of new traits during evolution is a major challenge in biology. RNS is observed in the orders Fabales, Rosales, Fagales, and Cucurbitales, which collectively constitute a monophyletic group known as the nitrogen-fixing clade within angiosperms. It is hypothesized that the common ancestor of these orders acquired the capability for RNS. To elucidate the evolutionary basis of RNS, our focus is on the NIN transcription factor, exclusive to nodulating plants and derived from NLP. While NIN retains some structural features of NLP, it has lost nitrate responsiveness and exhibits unique DNA binding properties. We have recently identified specific amino acid residues in NIN that could link its evolution to RNS capabilities. We aim to explore the relationship between the evolution of NIN/NLP transcription factor family and the capabilities of RNS using various plant species, which may lead to the development of a way to engineer RNS ability to currently non-nodulating plant species.
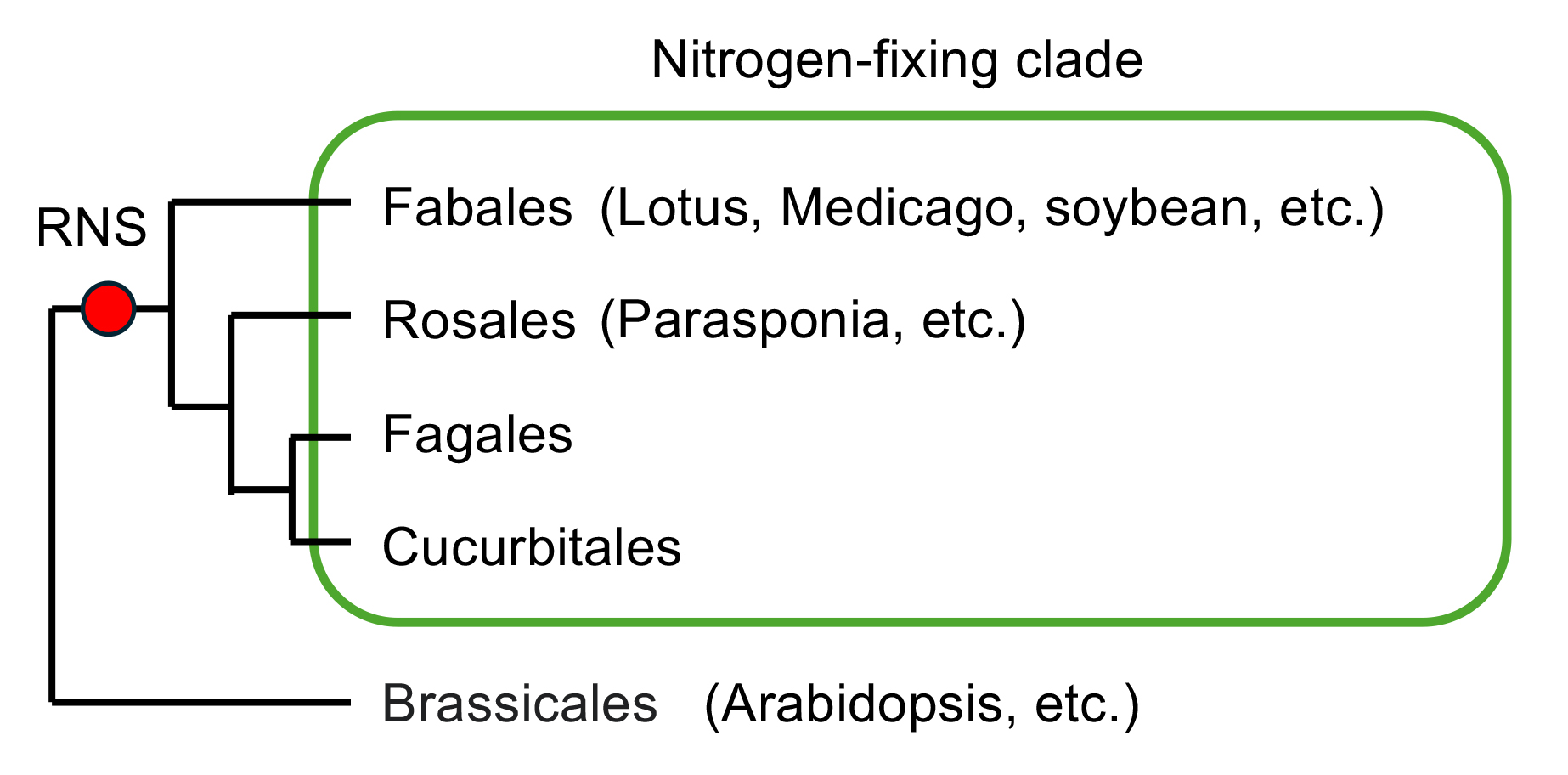
Plants capable of engaging in root nodule symbiosis are limited to the orders Fabales, Rosales, Fagales, and Cucurbitales, forming a nitrogen-fixing clade.
Iron supply mechanism to nodules
In RNS, plants provide an optimal environment for rhizobia, which includes supplying iron for their nitrogenase activity. Since rhizobia cannot synthesize iron, understanding how iron is delivered to nodules is crucial. We identified the IRON MAN (IMA) peptides, which are responsive to nitrogen status and are essential for nodule function. Knockout studies of LjIMA1 and LjIMA2 revealed impaired nodule formation and nitrogen fixation activity (Nature Commun 2024). Our research proposes an iron supply mechanism via IMA peptides, and we are investigating the details of how iron is transported to nodules.
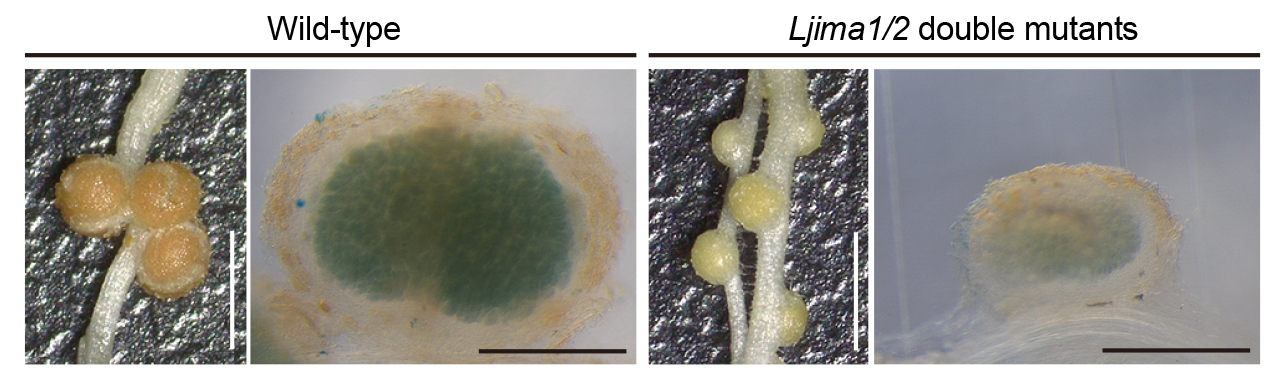
The Ljima1 Ljima2 double mutant forms more root nodules that are smaller in size compared to wild-type plants. When staining for iron within the nodules, the wild-type nodules appear blue, while the double mutant nodules show little to no blue staining, indicating a lower iron content. Scale bars: 2 mm (white), 500 µm (black).
Development of crops adapted to variable environments
Nitrogen fixation, crucial for converting atmospheric nitrogen into a usable form, has revolutionized food production through industrial methods. However, excessive nitrogen fertilizer use has led to environmental issues. With projections of adverse future conditions such as high temperatures and CO2 levels, existing crop varieties may not thrive. Our goal is to leverage RNS to address these global challenges. Supported by JST’s ALCA-Next project, we are developing resilient leguminous crops, like soybeans, that can effectively perform symbiotic nitrogen fixation under changing environmental conditions.
Contact Information
Takuya Suzaki, Ph.D.
Professor
Faculty of Life and Environmental Sciences
Tsukuba Plant-Innovation Research Center
University of Tsukuba
1-1-1 Tennodai, Tsukuba, Ibaraki, 305-8572, Japan
Tel: +81-29-853-4674
E-mail: suzaki.takuya.fn@u.tsukuba.ac.jp

